- Offerings
- Tools & Platforms
Tools & Calculators
- Open API
- Calculators
- SIP Calculator
- CAGR Calculator
- Compound Interest Calculator
- FD Calculator
- RD Calculator
- EPF Calculator
- Retirement Calculator
- HDFC SIP Calculator
- Mutual Fund Return Calculator
- Lumpsum Calculator
- Step Up SIP Calculator
- ETF SIP Calculator
- Brokerage Calculator
- Equity Margin Calculator
- SWP Calculator
- EMI Calculator
- MTF Calculator
- Pricing
- SKY Learn
- Mutual Funds
- Margin Trading
- Financial Planning
- Personal Finance
- Share Trading
- IPO
- Derivatives
- Currencies
- Intraday Trading
- Trading Strategies
- Demat Account
- Commodity
- ETF
Molbio Diagnostics IPO
To be Announced
Minimum Investment
IPO Details
TBA
TBA
TBA
TBA
TBA
NSE, BSE
TBA
TBA
Molbio Diagnostics IPO Timeline
Bidding Start
TBA
Bidding Ends
TBA
Allotment Finalisation
TBA
Refund Initiation
TBA
Demat Transfer
TBA
Listing
TBA
Molbio Diagnostics Limited
Molbio Diagnostics Limited is a point-of-care diagnostics company offering rapid, accurate, and affordable healthcare technologies for infectious and non-communicable diseases. Its patented ‘Truenat’ PCR platform, available in over 100 countries, enables decentralized diagnosis within an hour and supports molecular testing for 30 diseases, including TB, COVID, HIV, and Hepatitis. The ‘Truenat’ TB test is WHO-endorsed for rapid detection. Through subsidiaries and partners, the company also provides radiology, pathology, and breast health solutions. Operating five Indian facilities, it serves over 80 countries with 10,000+ devices sold.
Molbio Diagnostics Limited IPO Overview
Molbio Diagnostics Ltd. filed its Draft Red Herring Prospectus (DRHP) with SEBI on August 22, 2025, to raise funds through an Initial Public Offer (IPO). The IPO will be a Book Build Issue comprising a fresh issue worth ₹200 crore along with an offer for sale (OFS) of up to 1.26 crore equity shares. The equity shares are proposed to be listed on NSE and BSE, with Kfin Technologies Ltd. appointed as the registrar of the issue, while the book-running lead manager is yet to be announced. Important details such as IPO dates, price bands, and lot size remain undisclosed. The company’s promoters include Sriram Natarajan, Dr. Chandrasekhar Bhaskaran Nair, Sangeetha Sriram, Shiva Sriram, Sowmya Sriram, and Exxora Trading LLP.
Molbio Diagnostics Limited Upcoming IPO Details
| Category | Details |
| Issue Type | Book Built Issue IPO |
| Fresh Issue | ₹200 crore |
| Offer for Sale (OFS) | 1.26 crore equity shares |
| IPO Dates | TBA |
| Price Bands | TBA |
| Lot Size | TBA |
| Face Value | ₹1 per share |
| Listing Exchange | BSE, NSE |
| Shareholding pre-issue | 11,27,59,750 shares |
| Shareholding post-issue | TBA |
IPO Lots
| Application | Lots | Shares | Amount |
| Retail (Min) | TBA | TBA | TBA |
| Retail (Max) | TBA | TBA | TBA |
| S-HNI (Min) | TBA | TBA | TBA |
| S-HNI (Max) | TBA | TBA | TBA |
| B-HNI (Min) | TBA | TBA | TBA |
Molbio Diagnostics Limited IPO Reservation
| Investor Category | Shares Offered |
| QIB Shares Offered | Not more than 50% of the Offer |
| Retail Shares Offered | Not less than 35% of the Offer |
| NII (HNI) Shares Offered | Not less than 15% of the Offer |
Molbio Diagnostics Limited IPO Valuation Overview
| KPI | Value |
| Earnings Per Share (EPS) | ₹12.87 |
| Price/Earnings (P/E) Ratio | TBD |
| Return on Net Worth (RoNW) | 15.23% |
| Net Asset Value (NAV) | ₹84.51 |
| Return on Equity (RoE) | 16.20% |
| Return on Capital Employed (RoCE) | 20.98% |
| EBITDA Margin | 24.97% |
| PAT Margin | 13.48% |
| Debt to Equity Ratio |
Objectives of the IPO Proceeds
The Net Proceeds are intended to be utilised as per the details provided in the table below:
| Particulars | Amount (in ₹ million) |
| Funding capital expenditure towards the setting up of infrastructure for our research and development facility, Center of Excellence and connected office space. | |
| Funding capital expenditure towards the purchase of certain plant, machinery and other equipment for Goa Unit I, Goa Unit II and Visakhapatnam Unit | |
| General corporate purposes* | [●] |
Note: *To be determined upon finalisation of the Offer Price and updated in the Prospectus prior to filing with the RoC
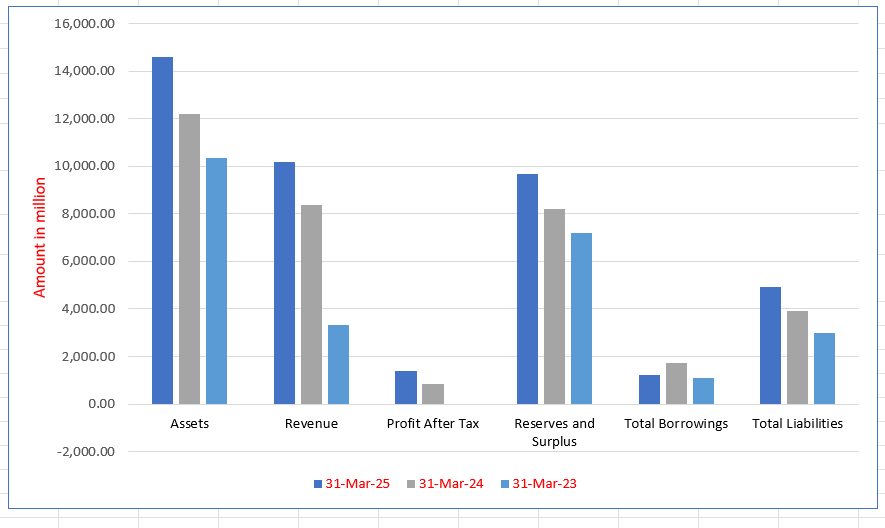
Molbio Diagnostics Limited Financials (in million)
| Particulars | 31 Mar 2025 | 31 Mar 2024 | 31 Mar 2023 |
| Assets | 14,615.55 | 12,210.56 | 10,342.11 |
| Revenue | 10,204.18 | 8,365.61 | 3,324.63 |
| Profit After Tax | 1,385.79 | 835.42 | (34.45) |
| Reserves and Surplus | 9,661.35 | 8,211.27 | 7,192.25 |
| Total Borrowings | 1,231.63 | 1,745.77 | 1,084.38 |
| Total Liabilities | 4,942.70 | 3,922.19 | 2,968.70 |
Financial Status of Molbio Diagnostics Limited
SWOT Analysis of Molbio Diagnostics IPO
Strength and Opportunities
- Portable, battery-operated Truenat platform enables PCR testing at point-of-care and in resource-limited settings
- Rapid diagnostics with results in under 60 minutes, aiding quick clinical decisions
- Broad multi-disease testing capability covering TB, influenza, malaria, hepatitis, etc.
- WHO endorsement for TB/RIF testing offers authoritative validation and adoption edge
- Proven domestic deployment with thousands of systems across India
- Expansion into radiology and AI-powered breast screening via acquisitions like Prognosys broadens product portfolio
- Global footprint across 80+ countries with 10,000+ devices and 40M+ tests enhances scale and brand
- Positioned well within a fast-growing POC molecular diagnostics market projected to double by 2032
- Made-in-India brand with strong public health collaborations aligns with policy support and trust
- Robust R&D through subsidiary Bigtec Labs supports continuous innovation
Risks and Threats
- Heavy reliance on point-of-care PCR devices—vulnerable if new, cheaper technologies emerge or if supply disruptions occur
- High R&D and manufacturing costs may strain margins or delay scaling
- Limited coverage of non-infectious diagnostics may limit market reach
- Market competition from global diagnostics firms (e.g., Roche, Thermo Fisher) could pressure market share
- Regulatory hurdles across different countries could slow international expansion
- Integration risk from acquisitions—managing new product lines and operations may be complex
- Dependence on third-party distributors may dilute control over sales, support, and brand experience
- Rapid technological evolution (e.g., CRISPR-based diagnostics) could render existing platforms less competitive
- Supply chain or manufacturing disruption (e.g., raw material shortages) could impact device availability
- Could face intellectual property or patent challenges—both domestically and internationally
Explore IPO Opportunities
Explore our comprehensive IPO pages to stay updated on the latest trends and insights.
About Molbio Diagnostics Limited
Molbio Diagnostics Limited IPO Strengths
Strong Position in Molecular Diagnostics
Molbio Diagnostics Limited is uniquely positioned to serve the rapidly expanding molecular diagnostics market through its Truenat platform. By offering accurate, rapid, and decentralized solutions for over 30 diseases, including TB, COVID, and HIV, the company meets rising healthcare demands. Its WHO-endorsed TB test highlights credibility, scale, and crisis-response capability.
Innovation-Driven R&D Capabilities
Molbio Diagnostics Limited prioritizes innovation with robust in-house R&D under subsidiary Bigtec, backed by over 13 years of continuous development. Its breakthroughs include India’s first Nipah virus test chip with emergency approval. With 114 scientists and strong patent holdings globally, the company leverages R&D to swiftly address gaps in diagnostics and sustain leadership.
Novel Portable Multi-Disease Platform
Molbio Diagnostics Limited has developed the portable, battery-operated Truenat system that delivers rapid molecular testing across multiple diseases in just 60 minutes. Its platform integrates nucleic acid extraction and real-time PCR, enabling accuracy, affordability, and flexibility. With wireless connectivity and ease of use, Truenat addresses diagnostic needs in both advanced laboratories and remote settings.
Scalable Model with Recurring Revenues
Molbio Diagnostics Limited operates a high-entry-barrier model with strong recurring revenue from proprietary Truenat test kits. Its closed ecosystem ensures continued demand post-device installation while expanding test offerings for infectious and non-communicable diseases. With growing adoption across India and global markets, the company benefits from scale, sustainability, and expanding diagnostic coverage.
Strategic Collaborations and Acquisitions
Molbio Diagnostics Limited enhances its capabilities through acquisitions like Prognosys in radiology and stakes in OptraScan for digital pathology. Collaborations with healthcare innovators expand its portfolio into breast health and host-biomarker diagnostics. These partnerships strengthen its ecosystem, extend reach into underserved markets, and position Molbio as a comprehensive diagnostic solutions provider globally.
Experienced Leadership Team
Molbio Diagnostics Limited is guided by an accomplished leadership team with decades of domain expertise. Led by CEO Sriram Natarajan, who earlier built Tulip Diagnostics into a global player, and CTO Chandrasekhar Nair, an award-winning innovator, the team brings proven track records in R&D, financial management, and global sales to drive sustainable business growth.
More About Molbio Diagnostics Limited
Molbio Diagnostics Limited is a pioneering point-of-care (POC) diagnostics company dedicated to expanding access to accurate, rapid, and cost-effective healthcare solutions. Its flagship innovation, the ‘Truenat’ platform, is a portable polymerase chain reaction (PCR) system designed for use in resource-limited settings, delivering decentralized diagnosis within an hour. As of March 31, 2025, Truenat is patented in more than 100 countries and offers molecular testing for 30 diseases through 42 assays, including tuberculosis (TB), COVID, Hepatitis B and C, HIV, and HPV.
Truenat Advantage
The platform operates in a high-entry-barrier market, having undergone 13 years of R&D to secure Indian Council of Medical Research (ICMR) certification. Notably, the Truenat TB test is:
- The only Indian-developed rapid molecular diagnostic for TB
- One of only two WHO-endorsed global tests for initial TB diagnosis and rifampicin resistance detection
Broader Solutions
In addition to molecular diagnostics, Molbio provides:
- Radiology systems through Prognosys
- Digital pathology tools via OptraScan
- Breast health screening solutions in collaboration with UE Lifesciences
Market Potential
The global point-of-care testing (POCT) market was valued at USD 27.83 billion in 2024 and is projected to grow at a CAGR of 18.74%, reaching USD 65.70 billion by 2029. With infectious diseases contributing to nearly one-third of global deaths in 2022, Molbio’s solutions are strategically positioned to address these critical healthcare challenges.
Technology and Design
The Truenat platform includes:
- Trueprep device: for automated nucleic acid extraction
- Truelab analyzer: available in UnoDx, Duo, and Quattro variants for simultaneous testing
- Truenat test kits: ready-to-use, room temperature-stable consumables
These innovations provide cost-effective, scalable, and portable testing options, especially valuable in underserved regions.
Global Reach and Growth
By March 2025, Molbio had deployed over 10,000 devices across 80+ countries, serving public health programs, hospitals, and laboratories. Revenues are driven by device sales and recurring income from proprietary test kits. Backed by strong R&D through Bigtec Labs and supported by global collaborations, Molbio continues to strengthen its innovation pipeline, expand its market presence, and set new standards in diagnostic accessibility.
Industry Outlook
The Indian Point-of-Care (POC) diagnostics market is witnessing robust growth, driven by the twin challenges of infectious and chronic diseases, coupled with the need for rapid, accessible testing—especially in rural and underserved areas.
Market Size & Growth
- POC Diagnostics (broad market): Valued at approximately USD 628 million in 2024, projected to reach USD 1.02 billion by 2030, at a CAGR of 8.45% (2025–2030).
- POC Molecular Diagnostics: Revenue of USD 298.6 million in 2024, expected to grow to USD 618.5 million by 2033, with an 8.5% CAGR.
- Rapid Diagnostics (broader category): Grew from USD 2.51 billion in 2024 to a forecasted USD 5.96 billion by 2035, at around 8.17% CAGR.
- Sepsis-specific POC segment: Expected to rise from USD 8.8 million in 2024 to USD 22.8 million by 2032, at a 12.6% CAGR.
Key Growth Drivers
- Rising prevalence of infectious diseases (TB, HIV, hepatitis) and non-communicable diseases (diabetes, cardiovascular disorders).
- Government initiatives to decentralise diagnostics and strengthen rural healthcare.
- Technological improvements such as miniaturisation, biosensors, microfluidics, AI integration, and smartphone compatibility.
- The COVID-19 pandemic accelerated adoption by proving the value of decentralised, rapid testing.
- Growth of mobile diagnostics, home-testing, and AI-assisted devices.
Segment Highlights & Trends
- The infectious diseases segment dominates the market due to high prevalence and urgent detection needs.
- Oncology and home-care modules are emerging as faster-growing sub-segments.
- Sepsis diagnostics show particularly high growth, reflecting urgent clinical demand.
Outlook for Molbio Diagnostics Limited
Molbio, with its Truenat PCR-based POC molecular diagnostics, is well-positioned in this fast-growing sector. Rising disease burden, strong government backing, demand for decentralised testing, and technological innovation provide strong tailwinds for expansion in India and globally.
How Will Molbio Diagnostics Limited Benefit
- Growing demand for rapid and accurate testing of infectious and chronic diseases directly supports Molbio’sTruenat platform, which already caters to TB, HIV, hepatitis, and more.
- Government programmes to strengthen decentralised diagnostics in rural India align perfectly with Molbio’s portable PCR systems, expanding its adoption.
- Strong CAGR projections in both POC diagnostics and molecular diagnostics ensure a larger revenue base and recurring kit sales.
- Rising need for affordable, point-of-care testing enables Molbio to position itself as a cost-effective and scalable solution provider.
- Increasing adoption of mobile and home-based diagnostics favours Molbio’s compact, easy-to-use devices.
- The surge in demand for rapid testing post-COVID has created lasting acceptance of decentralised diagnostic technologies, benefittingTruenat.
- Advances in AI, microfluidics, and biosensors open opportunities for Molbio to enhance product offerings and remain technologically competitive.
- Expanding oncology and sepsis diagnostics segments provide new growth avenues beyond infectious disease testing.
Peer Group Comparison
- There are no comparable listed companies in India that engage in a business similar to that of the company. Accordingly, it is not possible to provide an industry comparison as per the DRHP.
Key Strategies for Molbio Diagnostics Limited
Expanding Global Presence
Molbio Diagnostics Limited plans to scale its Truenat platform deployment across India and international markets. With devices already exported to over 80 countries, it aims to strengthen penetration in Africa and Southeast Asia, while entering Latin America, the US, and EU to capture rising molecular diagnostics demand.
Expanding Diagnostic Solutions Portfolio
Molbio Diagnostics Limited intends to broaden its test menu beyond the current 42 assays, adding 37 new assays for 22 diseases. By investing in R&D, sales, and marketing, and integrating imaging technologies, it seeks to provide comprehensive, multi-disease diagnostic solutions addressing both infectious and non-communicable diseases.
Developing New POC Platforms
Molbio Diagnostics Limited aims to leverage advanced multiplex PCR technologies to develop next-generation POC platforms. These solutions will address critical needs in immunochemistry, hematology, histopathology, antimicrobial resistance, and biochemistry. The strategy enhances its reach across both communicable and non-communicable diseases, positioning it as a leader in diversified molecular diagnostics.
Growth via Acquisitions and Alliances
Molbio Diagnostics Limited seeks inorganic growth through acquisitions, alliances, and its EDGE startup program. Past moves, including acquisitions in radiology and digital pathology, demonstrate portfolio expansion. Establishing a Bengaluru-based Center of Excellence by 2026 will accelerate innovation, co-development, and manufacturing, strengthening its ecosystem in India and global markets.
How to apply IPO with HDFC SKY?
Follow these simple steps to apply for an IPO through HDFC SKY. Secure your investments and explore new opportunities with ease by accessing the IPOs available on the platform.
1Login to your HDFC SKY Account
2Select Issue
3Enter Number of Lots and your Price.
4Enter UPI ID
5Complete Transaction on Your UPI App
FAQs On Molbio Diagnostics Limited IPO
How can I apply for Molbio Diagnostics Limited IPO?
You can apply via HDFCSky or other brokers using UPI-based ASBA (Application Supported by Blocked Amount).
What is the size of the Molbio Diagnostics Limited IPO?
The IPO comprises a fresh issue worth ₹200 crore and an Offer for Sale of 1.26 crore equity shares.
On which exchanges will Molbio Diagnostics shares be listed?
The equity shares of Molbio Diagnostics will be listed on both the Bombay Stock Exchange (BSE) and National Stock Exchange (NSE).
What is the primary objective of Molbio Diagnostics’ IPO?
The IPO aims to raise funds for expansion, research and development, acquisitions, and strengthening Molbio’s global diagnostics ecosystem.
Who are the promoters of Molbio Diagnostics Limited?
The company’s promoters include Sriram Natarajan, Dr. Chandrasekhar Bhaskaran Nair, Sangeetha Sriram, Shiva Sriram, Sowmya Sriram, and Exxora Trading LLP.